Evidence at your fingertips
Access real-time evidence for maternal and child vaccination
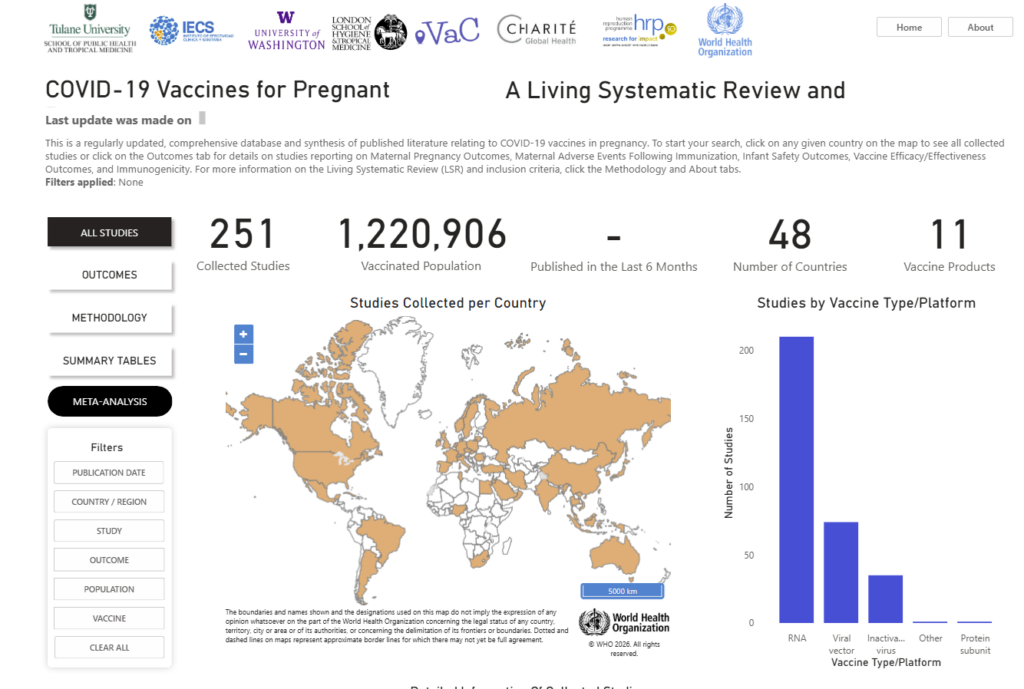
This page highlights a webinar demonstrating the Safe in Pregnancy / Safe in Children platform—an interactive hub that brings together real-time evidence through Living Systematic Reviews and meta-analyses on the safety, effectiveness, and immunogenicity of vaccines, with a focus on pregnant persons, children, and adolescents.
Registered participants will receive a calendar invite and a unique link to join the webinar.
Objectives
- Introduce Living Systematic Reviews and their value for vaccine safety and maternal–child health.
- Demonstrate the main features of the Safe in Pregnancy / Safe in Children platform.
- Provide hands-on examples for answering real-world clinical and policy questions.
- Foster discussion on how continuous evidence synthesis can support immunization programs and NITAGs.
Target audience
- Health professionals in maternal, neonatal, and child health
- Health decision-makers and NITAG members
- Researchers and academics in vaccine safety and epidemiology
- Students and trainees in evidence-based maternal and child health
Agenda (60 minutes)
- Welcome & Introduction (5 min) – Framing the importance of rapid access to maternal–child vaccine evidence
- Why Living Systematic Reviews? (10 min) – Overview of LSRs and their role in global health decision-making
- Platform demonstration (30 min)
- Searching evidence by disease and population
- Interpreting dashboards and outputs
- Case studies using the platform to inform vaccination decisions
- Q&A and next steps (15 min) – Open discussion and invitation to engage with the platform
Expected outcomes
- Increased awareness of Safe in Pregnancy / Safe in Children as a global public good
- Stronger understanding of LSRs as tools for rapid evidence translation
- Engagement of stakeholders interested in using the platform for teaching, policy, or practice
- Raised awareness of the importance of continuous monitoring of immunization safety
Speakers
- Agustín Ciapponi, MD, MSc, PhD Family physician and public health researcher specializing in systematic reviews and meta-analyses. Director of Cochrane Argentina and leader of multiple global evidence synthesis initiatives.
- Agustina Mazzoni, MD, MSc Obstetrician-gynecologist with extensive experience in epidemiology, health policy, and maternal health research, including large-scale trials and systematic reviews.
- Mabel Berrueta, MD, MSc Pediatrician, neonatologist, and epidemiologist with over 15 years of experience in maternal and child health research, implementation science, and living systematic reviews.
- Flor Muñoz, MD, MSc Flor M. Muñoz, MD, MSc, is an associate professor of pediatrics, infectious diseases, molecular virology and microbiology at Baylor College of Medicine and Texas Children’s Hospital in Houston, TX. Dr. Munoz is Chair of the institutional review board at Baylor College of Medicine. She is a physician-scientist with research activities focusing on the evaluation of vaccine safety and efficacy in special populations including pregnant women, children, and people with compromised immune systems, as well as the epidemiology and treatment of respiratory pathogens such as RSV, influenza, SARS-CoV2, and pertussis. Dr. Munoz is the lead for the Special Populations work package of the CEPI-SPEAC-Brighton Collaboration project.
Webinar materials
Materials from this session, including the recording and presentation slides, will be posted here following the webinar.








