


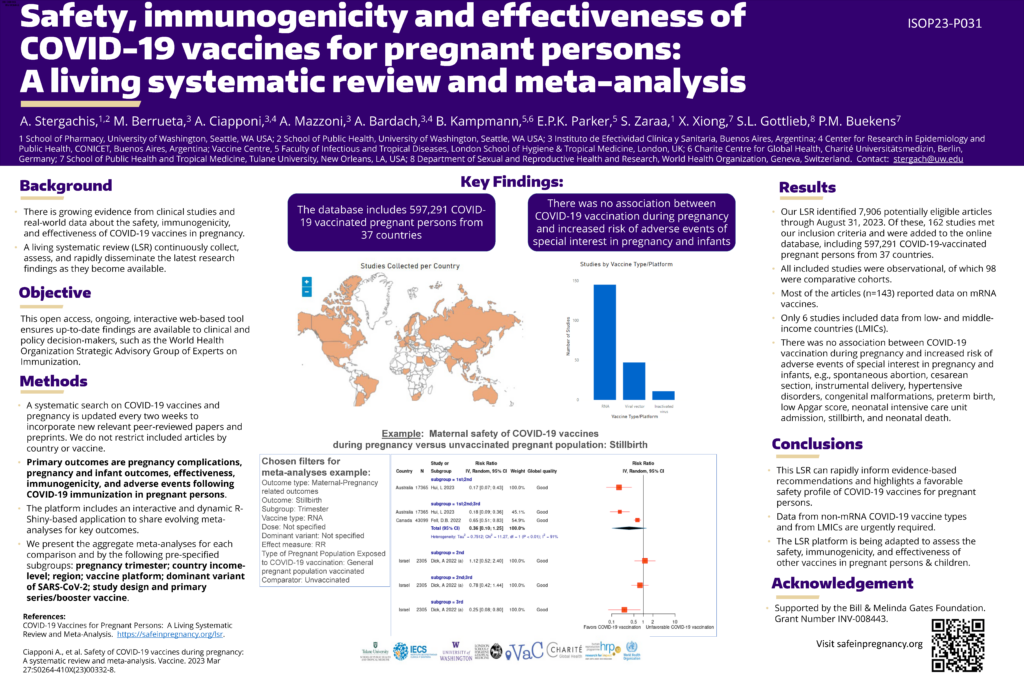
Dr. Andy Stergachis received the first-place poster award at the annual conference of the International Society of Pharmacovigilance (ISoP) in Bali, Indonesia in November 2023. He presented research on the safety, immunogenicity and effectiveness of COVID-19 vaccines for pregnant persons, which was funded by the Bill & Melinda Gates Foundation. Dr. Stergachis and team conducted a living systematic review and meta-analysis of nearly 600,000 pregnant persons who received COVID-19 vaccines.
Through rigorous assessment, the study authors found no increases in the risk of outcomes measured, which included spontaneous abortion, c-section, and congenital anomalies. They also reported on the effectiveness of COVID-19 vaccines in reducing hospital admissions for pregnant persons. The study authors designed the living systematic review platform, now available at safeinpregnancy.org, as a tool for decision-making.
The ISoP conference centered on research that contributes to improving patient safety worldwide in a meaningful way with the theme, “Putting Patients First In Pharmacovigilance: International Perspectives from Global South.” Some 150 posters were judged against the rigor of the methodology and the importance of the findings.
The potential for greater use and impact of the living systematic review platform lies in its navigability and timeliness. It is updated and easily searchable by many characteristics, including vaccine type and type of outcome. What’s more, the potential of this platform is not limited to COVID-19 vaccine data.
“The beauty of this work,” says Dr. Stergachis, “is that the living systematic review platform is being adapted to assess the safety, immunogenicity, and effectiveness of other vaccines in pregnant persons and children.”
A member of the study’s Advisory Board, Dr. Flor Muñoz, Associate Professor of Pediatrics-Infectious Disease at Baylor College of Medicine, leads SPEAC’s special populations work and the Maternal Immunization Working Group, of which Dr. Stergachis is a member. “There is no question that maternal immunization is a strategy with tremendous potential to improve the health of mothers and babies worldwide,” says Dr. Muñoz.
“The safeinpregnancy.org project and its living systematic review platform is an incredible tool to continue to assess not only the safety of vaccines administered during pregnancy, including COVID-19, but also the recently approved maternal RSV vaccine, and vaccines for emerging and epidemic pathogens that affect pregnant individuals or their infants,” says Dr. Muñoz.
Complementary work is being carried out through SPEAC’s special populations work stream, which is funded by the Coalition for Epidemic Preparedness Innovations (CEPI). The principal investigator of the living systematic review, Pierre Buekens of Tulane University, is engaged in this work alongside Dr. Muñoz. With funding from SPEAC and CEPI, the Safe in Pregnancy project now will address Chikungunya and Lassa fever vaccine safety in pregnancy and in children. A sister site, safeinchildren.org, will be launched in 2024.








